Abstract
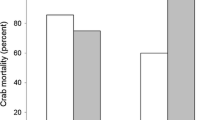
Landscape ecology, predominantly a terrestrial discipline, considers the effect of large-scale (tens of meters to kilometers) spatial patterns of habitats on ecological processes such as competition, predation, and flow of energy. In this study, a landscape-ecology approach was applied to a marine soft-sediment environment to examine rates of predation and transfer of secondary production in and around vegetated habitats. Seagrass beds naturally occur in a variety of spatial configurations from patches 1–10s of meters across with interspersed unvegetated sediments (i.e., patchy coverage) to more continuous coverage with little or no bare sediment. I designed experiments to address how percent coverage of seagrass in a 100-m2 area of seafloor, and the spatial arrangement (degree of patchiness or fragmentation) of an equal area (100 m2) of vegetation affected predation (lethal) and siphon nipping (sublethal) intensity on an infaunal bivalve, Mercenaria mercenaria (hard clam). Measures of seagrass density and biomass with different percent coverage of seagrass were also made. When clams were placed in both the vegetated and unvegetated portions of the seafloor nearly twice as many clams were recovered live with 99% seagrass cover than with 23% seagrass cover, while survivorship was intermediate with 70% cover. Cropping of clam siphons from both the vegetated and unvegetated sediments was also affected by the amount of seagrass cover in a 100-m2 area of seafloor: mean adjusted siphon weights were approximately 76% heavier from the 99% seagrass cover treatment than from the 70% or 23% cover treatments. Survivorship of clams placed within an equal area of seagrass in very patchy, patchy, and continuous spatial configurations was 40% higher in the continuous seagrass treatment than in either of the two patchy treatments. This study demonstrates that transfer of secondary production in the form of predation and cropping on an infaunal organism is altered as the percent cover of seagrass changes. While large-scale changes in the amount and spatial patterning of vegetation may affect habitat utilization patterns and foraging HGLoopbehavior, increased seagrass density and biomass with increased percent coverage of seagrass limit any conclusions concerning predator foraging behavior and feeding success in response to patch shapes and sizes. Instead, local changes in seagrass characteristics provide the most compelling explanation for the observed results.
Similar content being viewed by others
References
Andrén H (1992) Corvid density and nest predation in relation to forest fragmentation: a landscape perspective. Ecology 73: 794–804
Andrén H, Angelstam P, Lindström E, Widén P (1985) Differences in predation pressure in relation to habitat fragmentation: an experiment. Oikos 45: 273–277
Bell SS, Hicks GRF (1991) Marine landscapes and faunal recruitment: a field test with seagrass and copepods. Mar Ecol Prog Ser 73: 61–88
Bell SS, Hall MO, Fonseca MS (1994) Evaluation of faunal and floral attributes of seagrass beds in high and low energy regimes: a geographic comparison. In: Dyer KR, D'Elia C (eds) Changes in fluxes in estuaries: implications of science to management. Olson and Olson, London, in press
Blundon JA, Kennedy VS (1982) Refuges for infaunal bivalves from blue crab, Callinectes sapidus (Rathbun), predation in Chesapeake Bay. J Exp Mar Biol Ecol 65: 67–81
Brittingham MC, Temple SA (1983) Have cowbirds caused forest songbirds to decline? BioScience 33: 31–35
Coen LD, Heck KL Jr (1991) The interacting effects of siphon nipping and habitat on bivalve (Mercenaria mercenaria (L.)) growth in a subtropical seagrass (Halodule wrightii Aschers) meadow. J Exp Mar Biol Ecol 145: 1–13
Coen L, Heck KL, Abele LG (1981) Experiments on competition and predation among shrimps of seagrass meadows. Ecology 62: 1484–1493
Crowder LB, Cooper WE (1982) Habitat structural complexity and the interaction between bluegills and their prey. Ecology 63: 1802–1813
Currin BM, Reed JP, Miller JM (1984) Growth, production, food consumption, and mortality of juvenile spot and croaker: a comparison of tidal and nontidal nursery areas. Estuaries 7: 451–459
Danielson BJ (1991) Communities in a landscape: the influence of habitat heterogeneity on the interactions between species. Am Nat 138: 1105–1120
Duarte CM, Sand-Jensen K (1990) Seagrass colonization: patch formation and patch growth in Cymodocea nodosa. Mar Ecol Prog Ser 65: 193–200
Fonseca MS (1992) Restoring seagrass systems in the United States. In: Thayer GW (ed) Restoring the nation's marine environment. Maryland Sea Grant, College Park, MD, pp 79–110
Fonseca MS, Zieman JC, Thayer GW, Fisher JS (1983) The role of current velocity in structuring eelgrass (Zostera marina L.) meadows. Est Coast Shelf Sci 17: 367–380
Fonseca MS, Kenworthy WJ, Thayer GW (1988) Restoration and management of seagrass systems: a review. In: Hook DD et al. (eds). The Ecology and management of wetlands, vol. 2. Management, use and value of wetlands. Timber Press, Portland
Forman RTT (1990) Ecologically sustainable landscapes: the role of spatial configuration. In: Zonneveld IS, Forman RTT (eds) Changing landscapes: an ecological perspective. Springer New York, pp 261–280
Forman RTT, Godron M (1981) Patches and structural components for a landscape ecology, BioScience 31: 733–740
Forman RTT, Godron M (1986) Landscape ecology. Wiley, New York
Franklin JF, Forman RTT (1987) Creating landscape patterns by forest cutting: ecological consequences and principles. Landscape Ecol 1: 5–18
Gote RH, Gallagher EE, Scotts LE, Wilson KA (1981) Studies on decapod crustacea from the Indian River region of Florida. I. Community composition, structure, biomass and species-areal relationships of seagrass and drift algae-associated macrocrustaceans. Est Coast Shelf Sci 12: 485–508
Hartog C den (1971) The dynamic aspect in the ecology of seagrass communities. Thalassia Jugos 7: 101–112
Heck KL Jr, Thoman TA (1981) Experiments on predator-prey interactions in vegetated aquatic habitats. J Exp Mar Biol Ecol 53: 125–134
Heck KL Jr, Wetstone GS (1977) Habitat complexity and invertebrate species richness and abundance in tropical seagrass meadows. J Biogeogr 4: 135–142
Hines AH, Haddon AM, Wiechert LA (1990) Guild structure and foraging impact of blue crabs and epibenthic fish in a subestuary of Chesapeake Bay. Mar Ecol Prog Ser 67: 105–126
Irlandi EA, Peterson CH (1991) Modification of animal habitat by large plants: mechanisms by which seagrass influences clam growth. Oecologia 87: 307–318
Naveh Z, Lieberman AS (1984) Landscape Ecology: Theory and Application. Springer-Verlag, New York
Nelson WG (1979) An analysis of structural pattern in an eelgrass (Zostera marina) amphipod community. J Exp Mar Biol Ecol 39: 231–264
Orth RJ (1992) A perspective on plant-animal interactions in seagrasses: physical and biological determinants influencing plant and animal abundance. In: John DM, Hawkins SJ, Price JH (eds) Plant-animal interactions in the marine benthos (The Systematics Association Special Volume 46), Clarendon Press, Oxford, pp 147–164
Orth RJ, Heck KL Jr, Montfrans J van (1984) Faunal communities in seagrass beds: a review of the influence of plant structure and prey characteristics on predator-prey relationships. Estuaries 7: 339–350
Peterson CH (1982) Clam predation by whelks (Busycon spp.): experimental tests of the importance of prey size, prey density, and seagrass cover. Mar Biol 66: 159–170
Peterson CH, Quammen ML (1982) Siphon nipping: its importance to small fishes and its impact on growth of the bivalve Protothaca stamina. J Exp Mar Biol Ecol 63: 249–268
Peterson CH, Skilleter GA (1994) Foraging of Macoma balthica on deposited versus suspended foods: the role of siphon-cropping fishes. Oecologia (in press)
Peterson CH, Summerson HC, Duncan PB (1984) The influence of seagrass cover on population structure and individual growth rate of a suspension-feeding bivalve, Mercenaria mercenaria. J Mar Res 42: 123–138
Reise K (1978) Experiments on epibenthic predation in the Wadden Sea. Helgo Wiss Meeresunters 31: 51–101
Skilleter GA, Peterson CH (1994) Foraging of Macoma balthica on deposited versus suspended foods: complex interactions between competition and cropping. Oecologia (in press)
Small MF, Hunter ML (1988) Forest fragmentation and avian nest predation in forested landscapes. Oecologia 76: 62–64
Summerson HC, Peterson CH (1984) Role of predation in organizing benthic communities of a temperate-zone seagrass bed. Mar Ecol Prog Ser 15: 63–77
Sutherland JP, Karlson RH (1977) Development and stability of the fouling community at Beaufort, North Carolina. Ecol Monogr 47: 425–446
Thayer GW (ed) (1992) Restoring the nation's marine environment. Maryland Sea Grant, College Park, MD
Thayer GW, Kenworthy WJ, Fonseca MS (1984) The ecology of eelgrass meadows of the Atlantic coast: a community profile. US Fish and Wildlife Service FWS/OBS-84/02: 1–147
Trevallion A (1971) Studies on Tellina tenius da Costa, III. Aspects of general biology and energy flow. J Exp Mar Biol Ecol 7: 95–122
Trevallion A, Edwards RRC, Steele JH (1970) Dynamics of a benthic bivalve. In: Steele JH (ed) Marine food chains. University of California Press, Berkeley, pp 285–295
Turner MG (1989) Landscape ecology: the effect of pattern on process. Annu Rev Ecol Syst 20: 171–197
Vlas J de (1979) Secondary production by tail regeneration in a tidal flat population of lugworms (Arenicola marina) cropped by flatfish. Neth J Sea Res 13: 362–393
Woodin SA (1982) Browsing: important in marine sedimentary environments? Spionid polychaete examples. J Exp Mar Biol Ecol 60-35–45
Zonneveld IS, Forman RTT (eds) (1990) Changing landscapes: An ecological perspective. Springer, New York
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Irlandi, E.A. Large- and small-scale effects of habitat structure on rates of predation: how percent coverage of seagrass affects rates of predation and siphon nipping on an infaunal bivalve. Oecologia 98, 176–183 (1994). https://doi.org/10.1007/BF00341470
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00341470